2024 Mid-Year Review with Cameron Reynolds, Group CEO of Volition
- Share
- Tweet
- Share on Facebook
- Share
Ahead of Volition’s Annual Meeting of Stockholders next week, I am delighted to share our key highlights from the first half of 2024 and provide a preview of upcoming milestones.
Thank you to you all for your continued support in helping us to advance our mission to save the lives and improve outcomes of millions of people and animals worldwide through our novel epigenetics platform. This year we have successfully supported our veterinary licensing and distribution partners to launch our Nu.Q® Vet Cancer test and progressed both our Nu.Q® NETs product for sepsis and cancer detection technologies, to be ready for licensing. We have a large amount of study data that we expect is close to being ready for inclusion in our licensing data rooms, and interest from key industry players. Our focus in the second half of 2024 will include negotiating our first licensing deal in the human space. We intend to follow a similar licensing approach in the human space as we achieved in the veterinary space.
On behalf of the whole team at Volition, I am delighted to share the following recent key highlights:
Expanding Access to the Nu.Q® Vet Cancer Test
We have significantly expanded the availability of our Nu.Q® Vet Cancer Test; it is now available in 15 countries through global, regional and national veterinary diagnostic providers, including the world’s leading veterinary services companies: Antech, part of Mars Science and Diagnostics, IDEXX and most recently Fujifilm Vet Systems in Japan.
Establishing supply agreements with industry leaders is important for Volition, offering the potential to generate significant revenue and milestone payments for the Company. As previously reported, Volition has received $23 million in upfront and milestone payments to date relating to the exclusive agreement with Heska, an Antech company (“Heska”), providing in-hospital access to the Nu.Q® Vet Cancer Test.
Since April 2024, Antech has launched the test in several major markets: U.S., Canada, France, Italy, Germany, Spain, Australia and Hong Kong and is looking to expand into a number of other countries throughout the year.
Available in-clinic, at the point of care, the Nu.Q® Vet Cancer Test on the Element i+ analyzer is:
- Rapid (<10 mins)
- Accurate (detects 76% of systemic cancers at 97% specificity)
- Cost effective (only $35 to the vet)

These features allow the veterinarian to make informed clinical decisions quickly while the patient is still in the clinic.
Early feedback from the Antech team is encouraging and we are hopeful of a steady ramp up through the end of this year and into 2025.
In July 2024, following a soft launch in April, Fujifilm Vet Systems plans to launch nationwide in Japan and VetLab is also scheduled to launch in July in Poland, the Czech Republic and Slovakia.
In 2023, we sold 58,000 Nu.Q® Vet Cancer Tests (including components for Point-of-Care test), at an average revenue of $8/test supplied in 2023; we are aiming to triple the number of tests sold in 2024 and anticipate revenue growth as existing distributors gain momentum and new distributors come online.
Product Development
Led by Dr. Heather Wilson-Robles, the research and development team continues to progress research to expand the potential use of our Nu.Q® Vet technology to include:
- a cancer monitoring application to help determine Minimal Residual Disease and/or remission monitoring,
- helping to detect and potentially risk stratify sepsis in dogs, and
- the use of Nu.Q® Vet Cancer for both screening and monitoring in the feline population.
These use cases not only expand the clinical utility of Nu.Q® Vet but also create potential commercial opportunities, including a final milestone payment of $5 million relating to our exclusive agreement with Heska in connection with feline screening or monitoring.
Preparing the Data Rooms for Near-Term Licensing Opportunities
As stated in previous briefings, our commercial strategy is to monetize our Intellectual Property (“IP”) with upfront payments, milestone payments, royalties and sales of key components, much as we did with Nu.Q® Vet. We believe our Nu.Q® NETs product, Nu.Q® Lung and Capture-PCRTM technologies, have strong potential and we are seeking partners to collaborate with.
Volition seeks to drive innovation and conduct R&D in collaboration with our research partners and Centers of Excellence and commercialize our inventions via established global players, and regional and national companies to market, sell, and process our tests and technologies.
Our two underlying principles are:
- Low capital expenditures for our partners and low operating expenses for Volition.
- Low-cost and accessible tests worldwide.

We are in active commercial discussions with a number of companies including liquid biopsy, large diagnostic and IVD suppliers, some of which are significant players in sepsis, thrombosis, coagulation and oncology.
The data rooms to support commercial due diligence and negotiation regarding Nu.Q® Nets, Nu.Q® Cancer and Capture-PCR™ are in an advanced stage of preparation and are expected to open shortly. We intend to supplement the data rooms on an ongoing basis as, for example, more patents are filed / published / approved and further clinical data is signed off. Our focus across our product pillars is to license as broadly as possible with the aim to execute agreements in the future.
Nu.Q® NETs
Our focus is to develop a low-cost, routine test to stratify risk of sepsis, particularly those at risk of progressing to multiple organ failure; in addition to monitoring the disease progression and response to treatment.
Our previously published evidence demonstrates encouraging results for Nu.Q® NETs which were included in Su, Taccone et al’s recently published review article entitled “Circulating Nucleosomes as a Novel Biomarker for Sepsis”1.
Key outcome measures to demonstrate assay clinical utility (correlation with):
- Sepsis 3 definition
- Disease severity
- ICU mortality
- 28-day mortality
- Duration of organ support
- Length of stay (in ICU / and in-hospital)
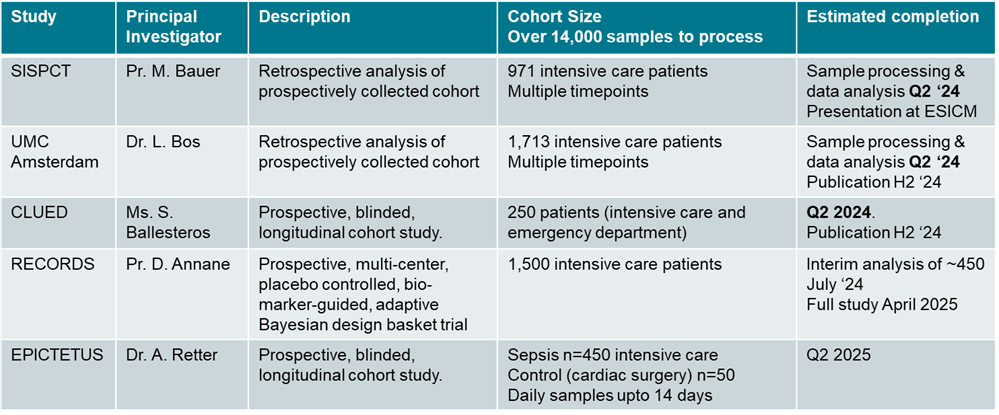
We have a number of extensive studies ongoing (see table below), with many large datasets expected to be available for the confidential data room this summer and publication anticipated either at the ESICM congress in October 2024 or in clinical papers.
Cancer
Our Nu.Q® Cancer research is largely focused on lung cancer, the leading cause of cancer related deaths. Our focus is to develop a low-cost, routine test to help:
- detect disease early,
- provide tailored treatment,
- assess response to treatment,
- identify Minimal Residual Disease, and
- support continued treatment decisions.
We have a range of studies from prospective / retrospective, blinded, longitudinal studies of lung cancer at diagnosis and during treatment / disease progression. Many of these studies have been completed and we anticipate opening our confidential data rooms shortly, with further publications expected beginning in the third quarter.
We are in discussions with a number of National Screening programs and with our Centers of Excellence to utilize the technology in clinical use as early as January 2025.

Capture-PCR™
In October 2023, we unveiled what we believe to be a very exciting, new breakthrough cancer detection method at the annual congress of the European Society for Medical Oncology. We now call this Capture-PCRTM.
Blood tests that can detect cancer at an early stage before the cancer spreads to form secondary tumors are the goal of academic and commercial research worldwide. However, detection of cancer-derived circulating tumor DNA (“ctDNA”) in the blood is challenging because it may comprise less than 0.1% of the DNA present among a background of 99.9% normal DNA that has almost the same sequence.
This means that ctDNA cannot be measured directly. Current liquid biopsy methods address this challenge by extracting and sequencing all circulating DNA – both healthy and cancer – and then use complex bioinformatics to analyze the healthy and cancer derived DNA sequence data to determine indirectly whether any ctDNA was present in the blood sample. This is extremely expensive and takes about two weeks.
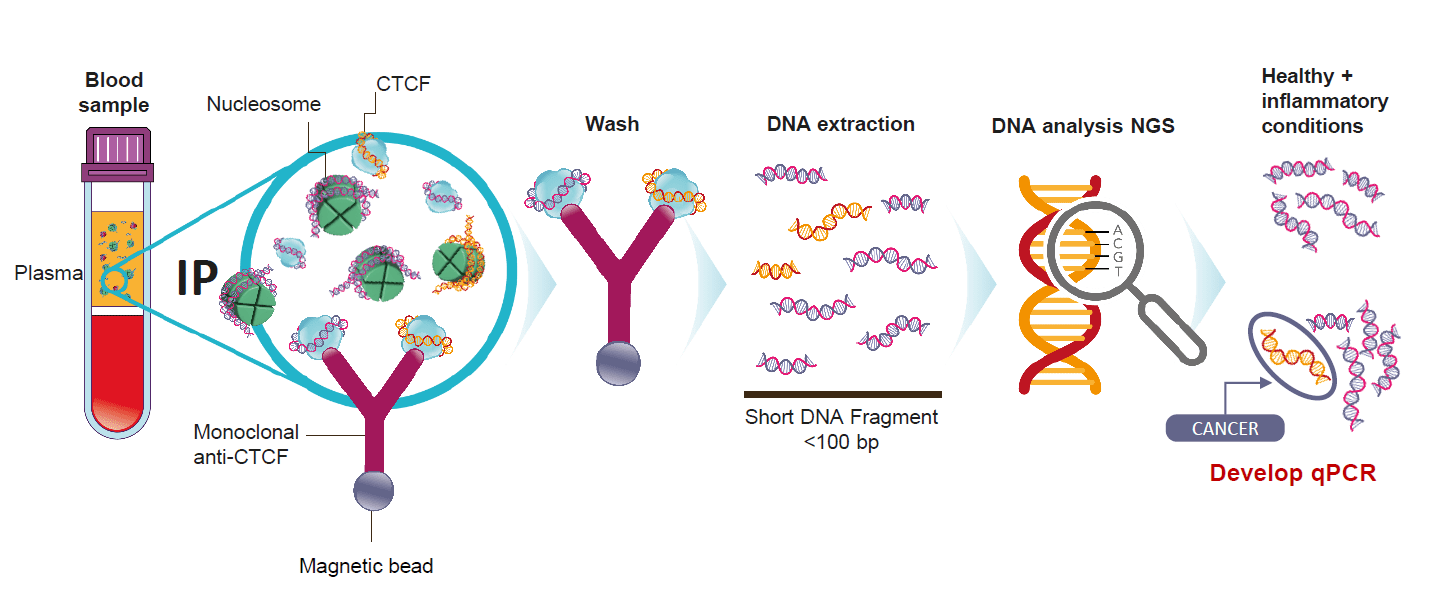
We believe direct detection of ctDNA would be low cost and much easier, but this is only possible if cancer-derived and normal circulating DNA could be separated. However, this has not previously been achieved.
We believe we have discovered a previously unknown class of ctDNA biomarkers that are invisible to current liquid biopsy methods. These new DNA biomarkers can be separated on the basis of the different proteins they are bound to in the blood of cancer patients and we have developed a novel direct liquid biopsy method for their analysis. We believe this new liquid biopsy assay is the first ever reported method able to physically separate a class of pure cancer-derived ctDNA fragments from normal DNA fragments in the blood. Once isolated from all normal DNA, cancer derived ctDNA fragments are detected directly with a simple, rapid, low-cost PCR test.
We believe this could be a breakthrough method which could obviate expensive, time-consuming DNA sequencing and bioinformatics thus allowing for rapid, cost-effective detection in a routine blood test and has the potential to significantly change liquid biopsies.
We have identified hundreds of potential short cfDNA biomarker sequences using samples from six cancer types and thus far we have developed prototype qPCR assays to only 14 sequences and tested them in a small number of samples.
We then performed a small study that demonstrates that the method concentrates ctDNA fragment sequences to near 100% purity and discriminates early-stage cancer. We plan to submit this method and these results for publication in a peer-reviewed journal in July 2024.
Our commercial strategy is to license this technology and we have been very encouraged by the level of interest shown by a wide range of companies.
Summarizing Thoughts
We believe we have made great progress in our mission throughout the first half of 2024 with some fantastic achievements by our whole team across the different product pillars. We have broadened and strengthened our Intellectual Property portfolio and we believe we have several potentially significant opportunities that are now ready for commercialization through licensing.
We have established plans in place for the remainder of this year and beyond. We have developed a very broad range of tests and technologies and our focus now is on commercialization in the human space following the licensing model we developed successfully with Nu.Q® Vet.
Thank you all again for your interest in Volition.
References
- Su, F.et al, Biomedicines 2024. https://doi.org/10.3390/biomedicines12071385
Safe Harbor Statement
Statements in this Business Review may be “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that concern matters that involve risks and uncertainties that could cause actual results to differ materially from those anticipated or projected in the forward-looking statements. Words such as “expects,” “anticipates,” “intends,” “plans,” “aims,” “targets,” “believes,” “seeks,” “estimates,” “optimizing,” “potential,” “goal,” “suggests,” “could,” “would,” “should,” “may,” “will” and similar expressions identify forward-looking statements. These forward-looking statements relate to, among other topics, Volition’s expectations related to the launch of product sales with counterparties to agreements, the success of negotiations and the timing, completion and execution of term sheets and/or agreements with third parties regarding the licensing and distribution of Volition’s products, the timing, completion, success and delivery of data from clinical studies and related publications, the potential uses, benefits and effectiveness of its Nucleosomics™ technology platform, including the Nu.Q® NETs test, the Nu.Q® Vet Cancer Test, Capture-PCR™, revenue growth, and the timing and execution of Volition’s strategy with the FDA. Volition’s actual results may differ materially from those indicated in these forward-looking statements due to numerous risks and uncertainties, including, without limitation, results of studies testing the efficacy of its tests, a failure by the marketplace to accept Volition’s Nu.Q® NETs test, Nu.Q® Vet Cancer Test or other products based on its Nucleosomics™ platform; Volition’s failure to secure adequate intellectual property protection; Volition’s failure to obtain necessary regulatory clearances or approvals to distribute and market future products; Volition will face fierce competition and its intended products may become obsolete due to the highly competitive nature of the diagnostics and disease monitoring markets and their rapid technological change; downturns in domestic and foreign economies; and other risks, including those identified in Volition’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as well as other documents that Volition files with the Securities and Exchange Commission. For instance, if Volition fails to develop and commercialize diagnostic, prognostic or disease monitoring products, it may be unable to execute its plan of operations. Forward-looking statements are based on current expectations, estimates and projections about Volition’s business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Forward-looking statements are made as of the date of this Business Review, and, except as required by law, Volition does not undertake an obligation to update its forward-looking statements to reflect future events or circumstances.
Nucleosomics™, Nu.Q® and Capture-PCR™ and their respective logos are trademarks and/or service marks of VolitionRx Limited and its subsidiaries. All other trademarks, service marks and trade names referred to in this Business Review are the property of their respective owners. Additionally, unless otherwise specified, all references to “$” refer to the legal currency of the United States of America.

Subscribe for Volition product updates.
Or, click here for Investor Updates and press releases.
"*" indicates required fields