-
Nu.Q® Technology
Our technology detects characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer, sepsis and other diseases.
-
- Our Tests
- Human Health
-
Nu.Q® NETs
Nu.Q® NETs is a groundbreaking CE-marked diagnostic solution that clinicians can use to detect NETosis.
-
Nu.Q® Discover
Buy our Nu.Q® Discover H3.1 Research Use Only Assay
- Animal Health
-
Nu.Q® Vet Cancer Test
Nu.Q® Vet Cancer Test detects 76% of systemic cancers at 97% specificity.

Early cancer detection by plasma CTCF transcription factor analysis
- Share
- Tweet
- Share on Facebook
- Share
In October 2023, we presented what we believe to be a breakthrough cancer detection method at the annual congress of the European Society for Medical Oncology.
In this article, we provide more insight into our exciting new liquid biopsy blood test method for early-stage cancer.
For further details, you can also watch our webinar, featuring Dr. Jake Micallef, our Chief Scientific Officer, Dr. Andrew Retter, an NHS Consultant, and Gael Forterre, our Chief Commercial Officer.
OVERVIEW
In early-stage cancer, it is difficult to detect cancer-derived circulating tumor DNA (ctDNA) in the blood because it may comprise only 0.01% of the DNA present among a background of 99.99% normal DNA. Moreover, most of the cancer DNA has exactly the same sequence as normal DNA.
Current ctDNA detection methods involve DNA extraction, sequencing of all (cancer and normal) circulating DNA and analysis of the sequencing data using sophisticated computer bioinformatics to tell them apart. Physical separation of tumor-derived and healthy circulating DNA had previously never been reported.
OUR SOLUTION
We have developed a novel method for liquid biopsy involving the first reported physical isolation of a class of tumor-derived ctDNA fragments from blood. Cancer-derived ctDNA fragments are then extracted after removal of all normal background DNA of the same sequence, for detection with a simple, low cost PCR test.
Our breakthrough method obviates expensive, time-consuming DNA sequencing and bioinformatics – allowing for rapid, cost-effective detection in a routine blood test. It may also be suitable for automation, enabling application in hospital laboratories.
WORKFLOW
Based on over a decade of work on the chemistry of circulating chromatin fragments, we have developed a transformational wet chemistry pathway that identifies and physically isolates chromatin fragments that we know are tumor-derived from background DNA of the same sequence, using Chromatin Immunoprecipitation (ChIP).
Quantitative real-time PCR (qPCR) testing is undertaken to establish whether cancer is present.
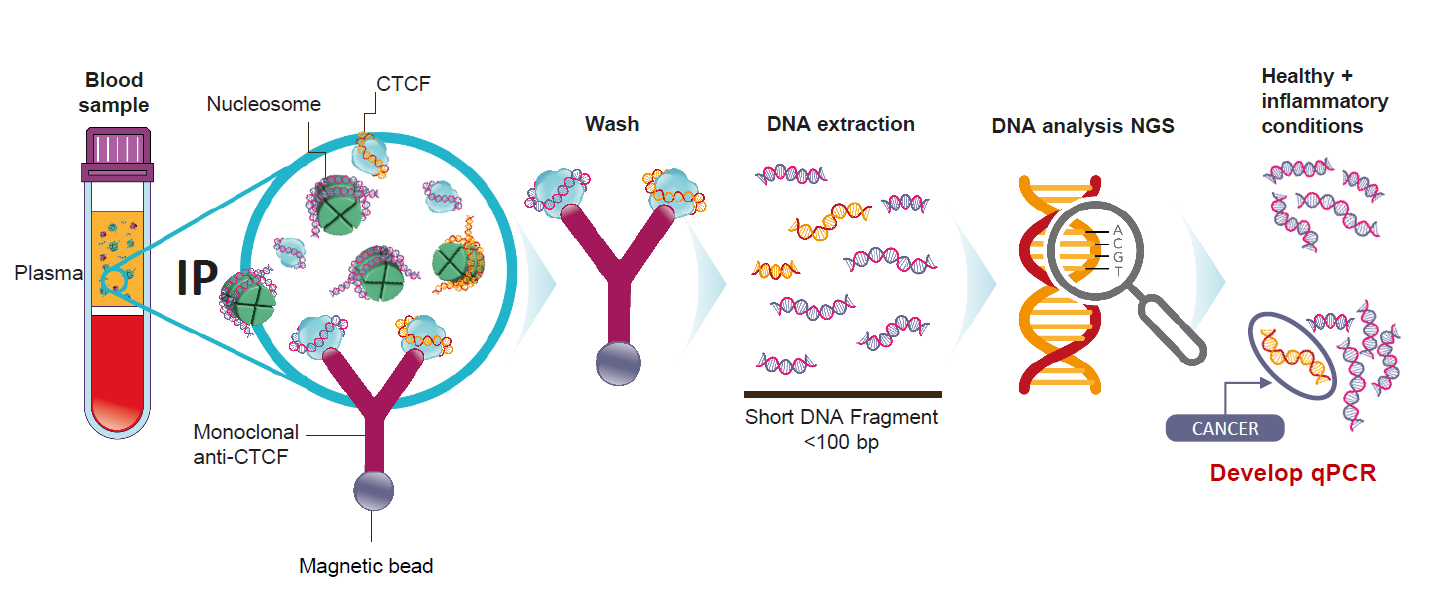
APPLICATION
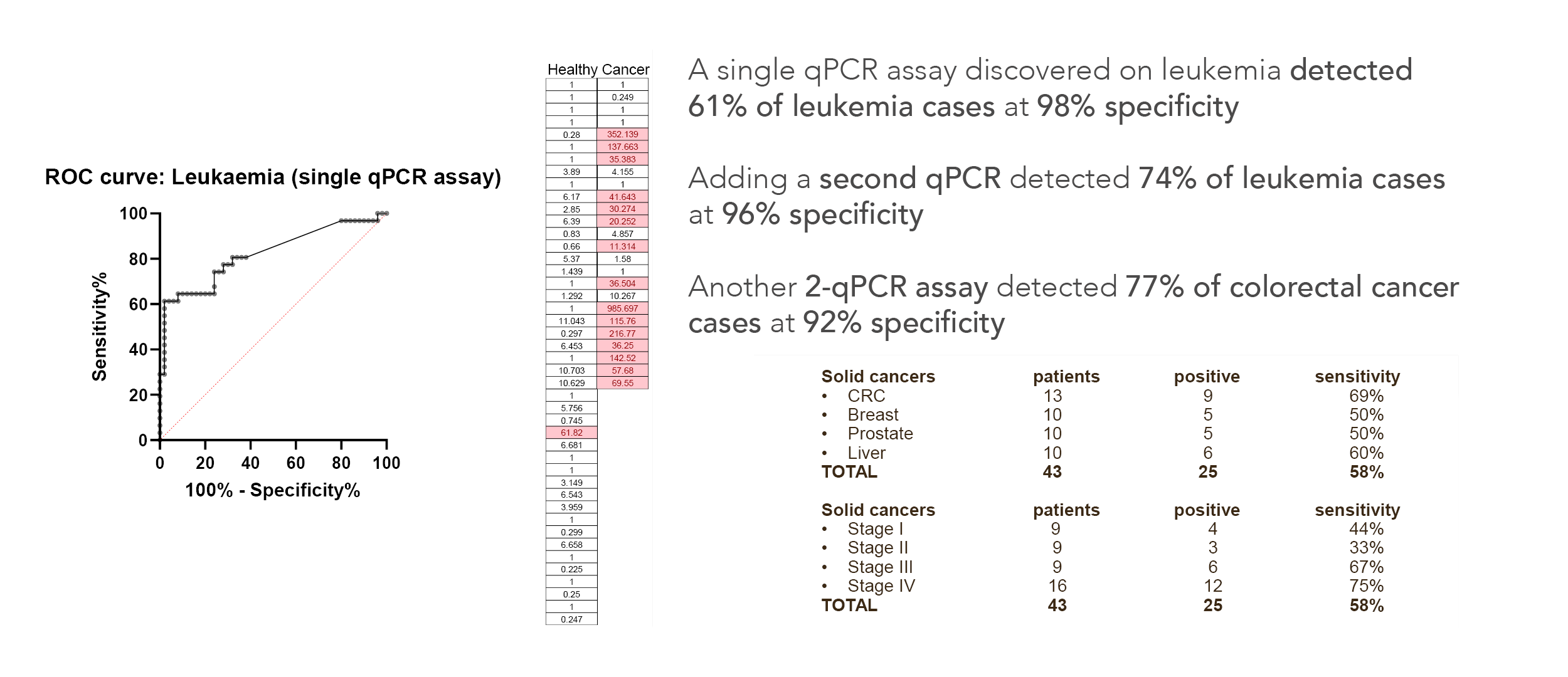
We tested the new method in a first small clinical experiment and detected a range of liquid and other cancers, including at early-stage I disease. [include data tables]
74% of leukemias were detected at 96% specificity with a single qPCR assay.
77% of colorectal cancers were detected at 92% specificity with 2-qPCR assays.
These early assays were developed using a leukemia model, but surprisingly also detected many other cancers including detecting colorectal cancer in a blood test with an accuracy approaching that of current Fecal Immunochemical Tests (FIT).
OUR NEXT STEPS
Results to date are exciting and may pave the way for a whole new class of undiscovered biomarkers, with hundreds or thousands of possible targets.
We are now developing a range of cancer-specific assays which we expect to be more accurate and look forward to sharing our progress throughout 2024 and beyond.
Our commercial strategy is to license this proprietary technology to an industry leader/leaders.
“The novel CTCF-ChIP/qPCR method developed by Volition shows great promise for the accurate, rapid, low-cost detection of cancer. The test complements existing approaches and has the potential to reach the high levels of sensitivity and specificity required to detect early-stage disease. It may also be suitable for automation, enabling application in hospital laboratories. We look forward to working with Volition on further research and development in this important and exciting area.”

Dr Michael Hubank
Professor of Translational Genomics at the Institute of Cancer Research, and Scientific Director of Clinical Genomics at The Royal Marsden NHS Foundation Trust
Subscribe for Volition product updates.
Or, click here for Investor Updates and press releases.
"*" indicates required fields