- Nu.Q® Technology
Our technology detects characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer, sepsis and other diseases.
-
- Our Tests
- Human Health
- Nu.Q® NETs
Nu.Q® NETs is a groundbreaking CE-marked diagnostic solution that clinicians can use to detect NETosis.
- Nu.Q® Discover
Buy our Nu.Q® Discover H3.1 Research Use Only Assay
- Animal Health
- Nu.Q® Vet Cancer Test
Nu.Q® Vet Cancer Test is an affordable, accessible blood test that detects cancer in dogs.

Our Nu.Q® NETs assay detects diseases associated with NETosis, such as sepsis.
Rapid detection of sepsis is vital.
Sepsis is the number one cause of death in hospitals worldwide. It kills an estimated 11 million people a year, which is more than cancer or coronary disease. In 2017, there were an estimated 49 million cases, with over half of all cases occurring among children and accounting for 2.9 million deaths in under-fives. Just under half of all survivors are left with psychological and/or physical effects.
Sepsis, also known as ‘blood poisoning’, is hard to identify. Initial symptoms of sepsis are difficult to distinguish from most infections and there is currently a lack of tests to diagnose it symptomatically. Without prompt treatment, it can lead to multiple organ failure and death.
Early detection and treatment of sepsis has the potential to improve survival – and improve the quality of life of survivors. Imagine if a simple blood test could help diagnose sepsis and identify those patients more likely to deteriorate.
Our Nu.Q® NETs assay detects diseases associated with NETosis, such as sepsis. It has the potential to help doctors accurately diagnose disease and could also help predict disease severity, measure the treatment response and monitor disease progression.
This simple, low-cost, accessible, routine blood test quantifies an individual’s level of circulating H3.1 nucleosomes, a surrogate marker for NETs. An elevated level of NETs can lead to tissue damage, and in severe cases, sepsis, organ failure and death.
Estimated 11 million deaths from sepsis worldwide each year
Risk of death increases by 7.6% for every hour of treatment delay.
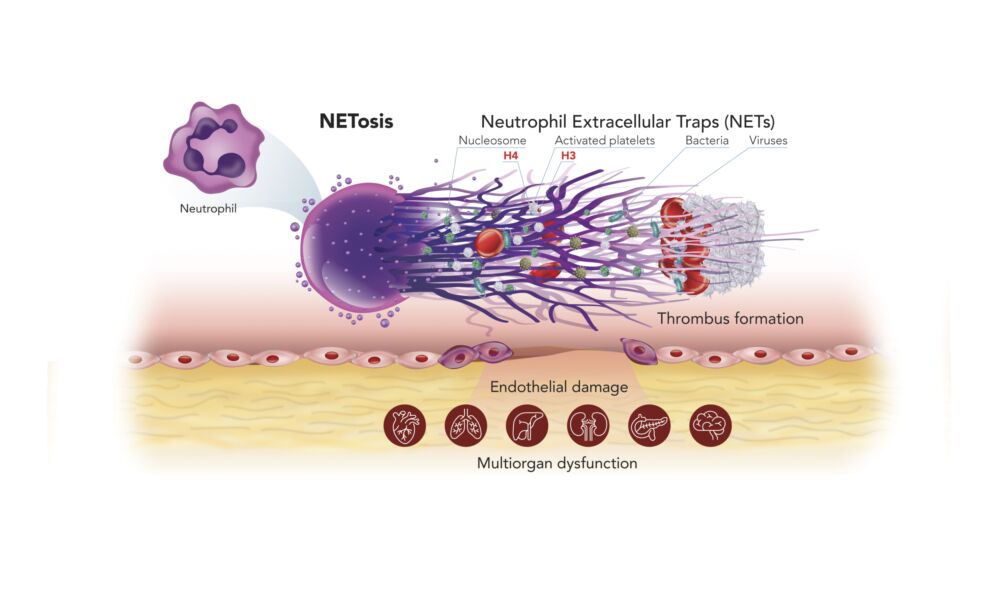
Understanding NETs and NETosis – a recent breakthrough in medical science

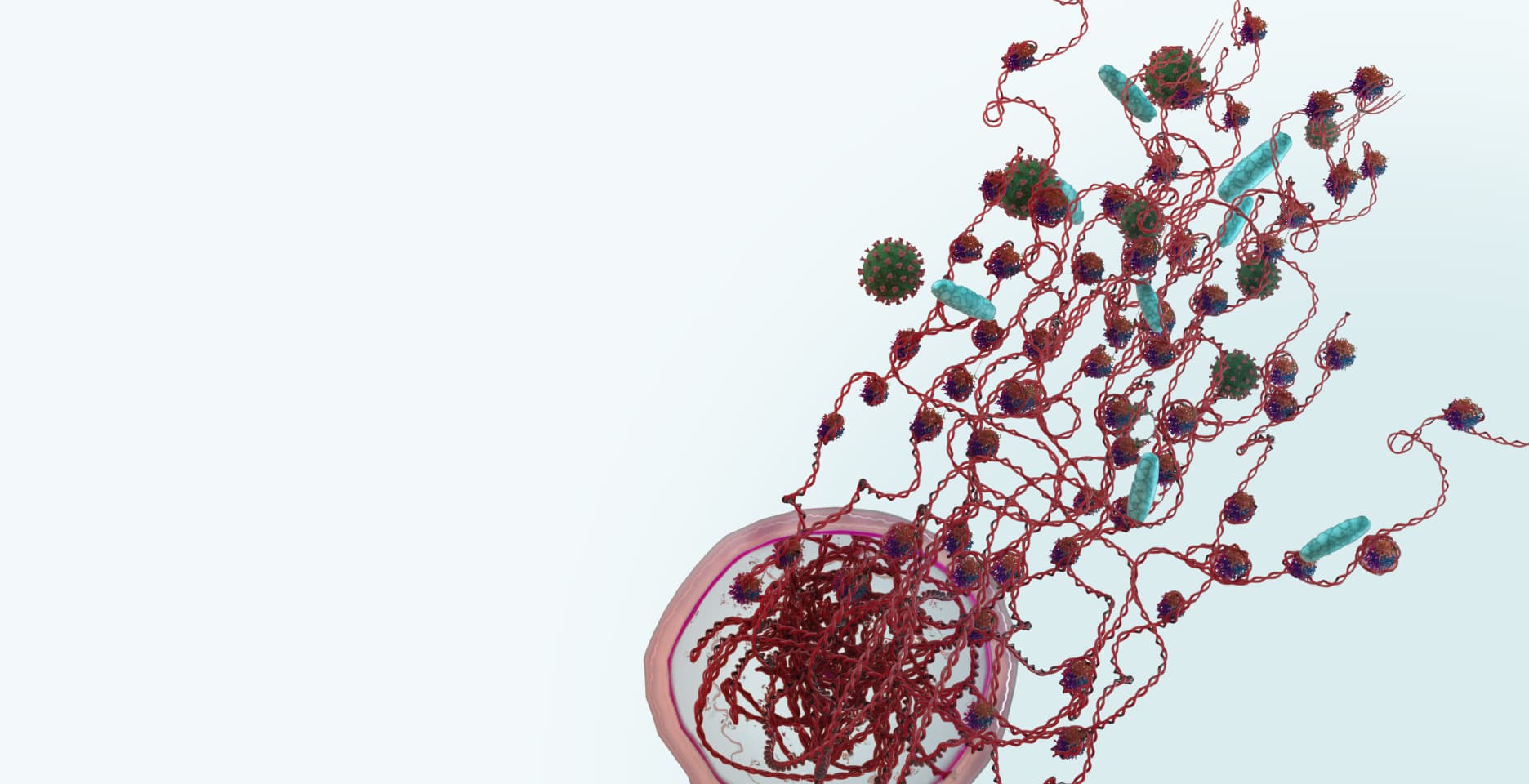
The immune system is comprised of many different types of white blood cell with different functions. The most abundant cells are neutrophils, which serve as the first line of defense. When they detect bacteria, viruses or fungi, these cells produce Neutrophil Extracellular Traps (NETs) – sticky webs made of long strings of nucleosomes that stop the threat from spreading around the body. This unique form of cell death is called NETosis. NETs released into the blood stream, contain nucleosomes, which can be detected by our Nu.Q® NETs assay – the only analytically validated assay to quantify the level of NETs.
Although NETs play a critical role in our normal immune response, elevated levels of NETs are a complicating factor associated with poor patient outcomes in sepsis, cancer, and a range of other diseases.
Our Solution
Introducing Nu.Q® NETs.
Nu.Q® NETs is a groundbreaking CE-marked diagnostic solution that clinicians can use to detect NETosis. Our assay can be used to identify patients with clinically relevant elevated levels of circulating NETs and enable physicians to rapidly treat these patients.
Chromosomes and NETs are made of nucleosomes, and NETs play a pivotal role in endothelial damage and the formation of microthrombi and resultant multiorgan failure.
Our Nu.Q® NETs is the only analytically validated assay to quantify the level of NETs. It is platform agnostic so it can be adapted to any workflow/clinical setting – including central lab and point of care.
Nu.Q® NETs will support clinical decision-making, enabling physicians to act quickly, improving patient outcomes and patient management.
It is registered for use in Europe in an automated ChLIA (Chemiluminescence immunoassay) format.
Simple, routine blood test
Correlates with SOFA, APACHE II
The only analytically validated assay to quantify the level of NETs
Use cases – key findings to date

-
We are collaborating with centres of excellence worldwide to ensure Nu.Q® NETs can be introduced effectively into clinical settings. Findings to date show:
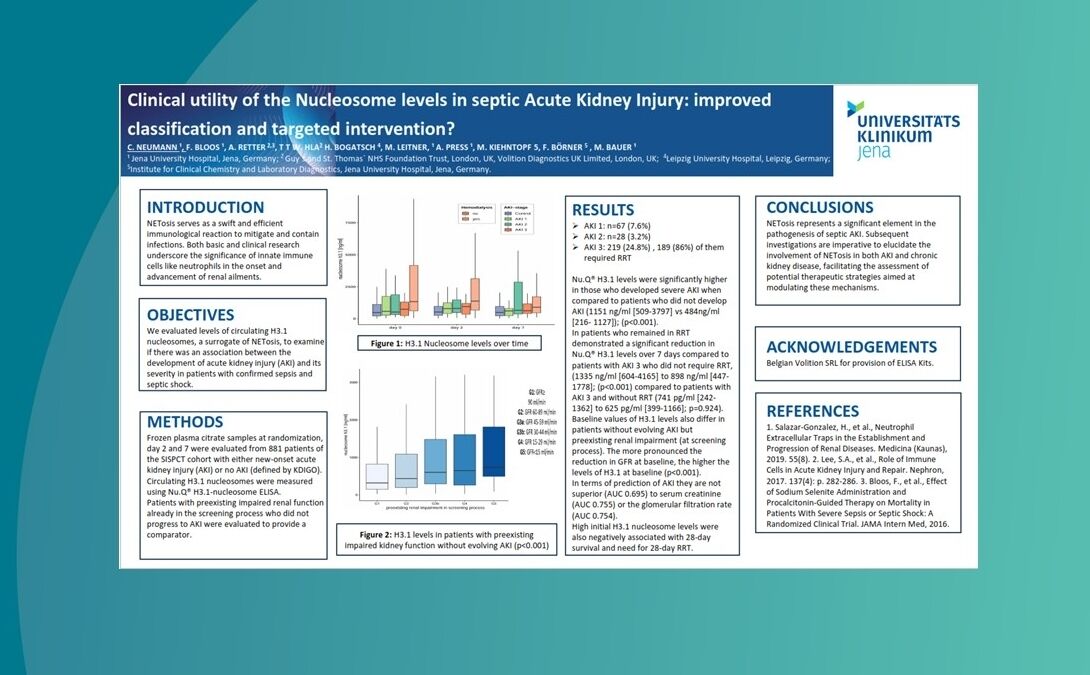
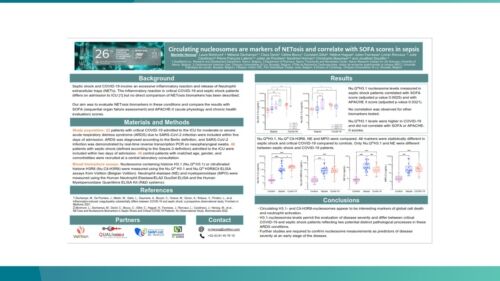
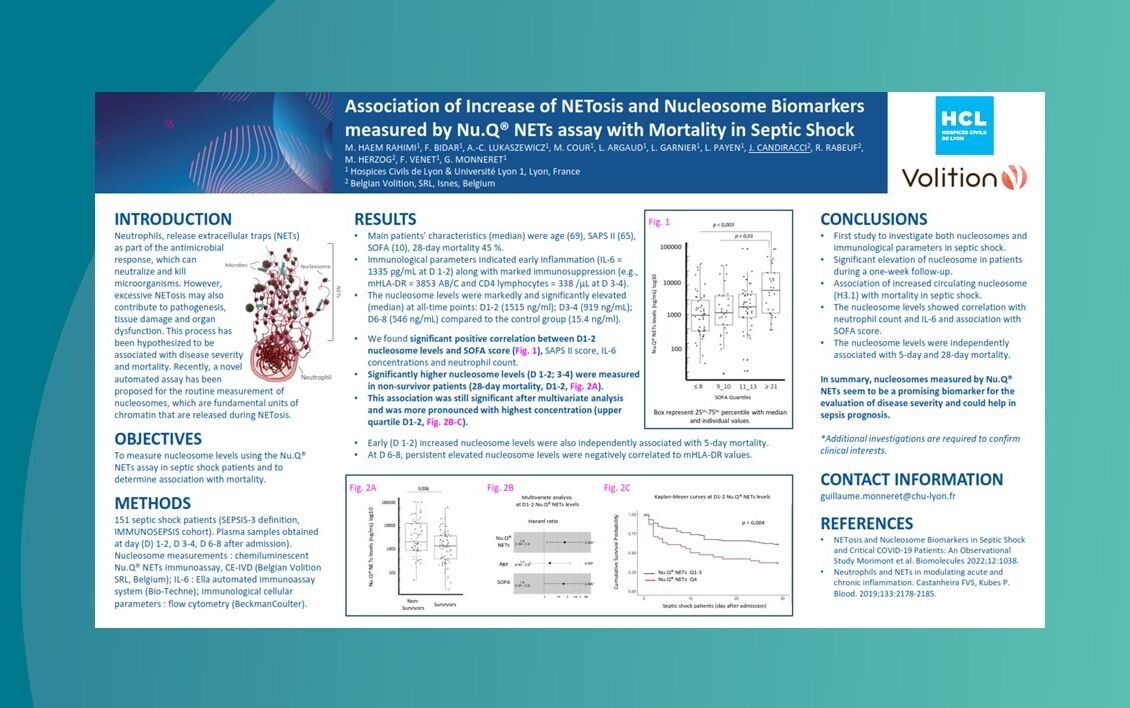
Nu.Q® NETs could be used to predict patients at greater risk of deteriorating from sepsis.
Nu.Q® NETs levels in ICU patients with sepsis correlate with SOFA score.
Collaboration
Working together.
We are working with teams at several major hospitals across Europe, undertaking studies to test our technology as a diagnostic aid for sepsis, to monitor disease progression and treatment response.
Detect NETosis
Predict disease severity and support risk stratification
Monitor disease progression
Professor of Medicine at University Paris Saclay-UVSQ
Brochure & Clinical Papers
Contact Nu.Q® NETs Team
Modal Contact Form (NETS)
"*" indicates required fields