- Nu.Q® Technology
Our technology detects characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer, sepsis and other diseases.
-
- Our Tests
- Human Health
- Nu.Q® NETs
Nu.Q® NETs is a groundbreaking CE-marked diagnostic solution that clinicians can use to detect NETosis.
- Nu.Q® Discover
Buy our Nu.Q® Discover H3.1 Research Use Only Assay
- Animal Health
- Nu.Q® Vet Cancer Test
Nu.Q® Vet Cancer Test is an affordable, accessible blood test that detects cancer in dogs.
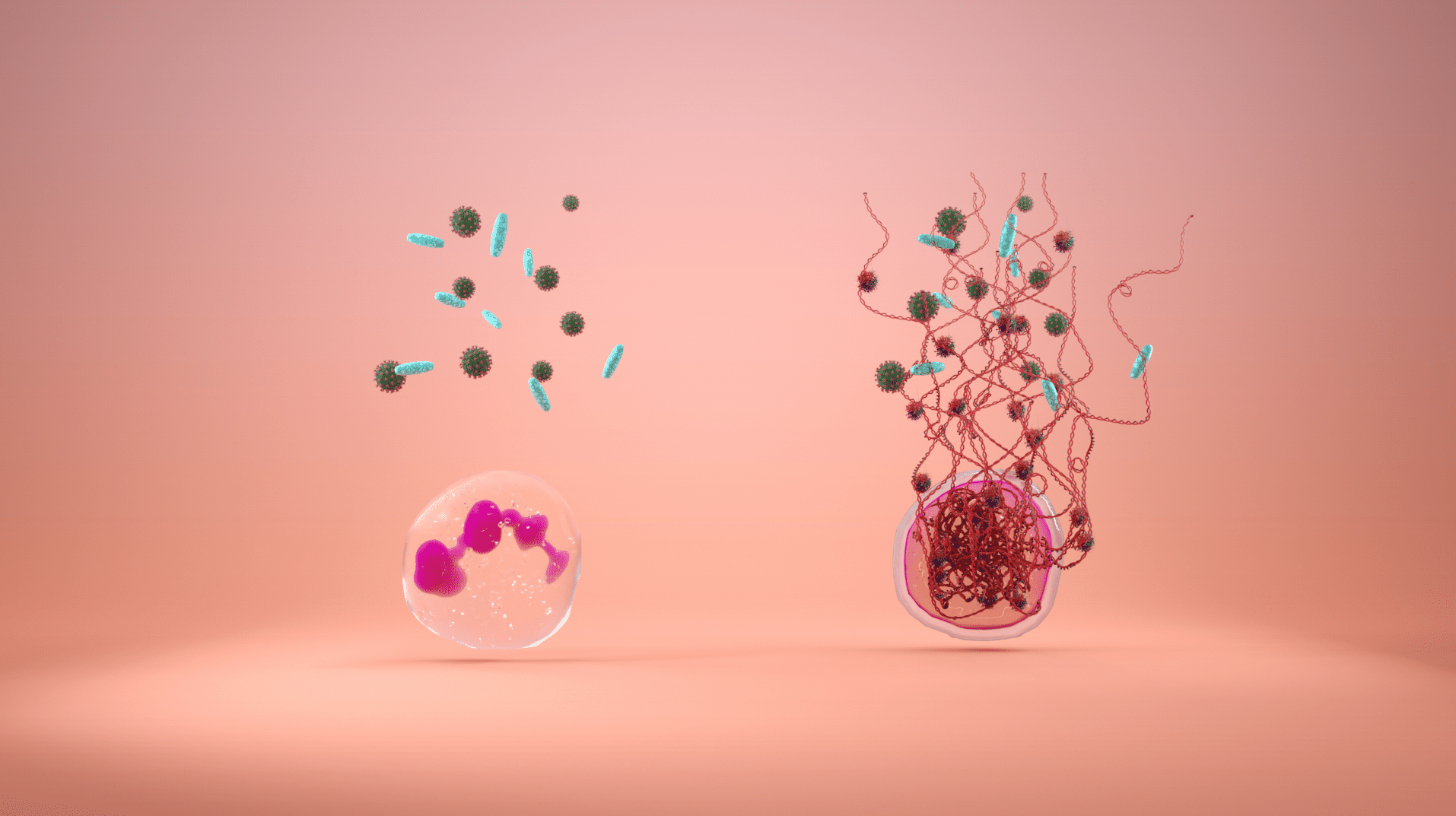
Volition Announces U.S. Clinical Study for NETs
- Share
- Tweet
- Share on Facebook
- Share
HENDERSON, Nev., Aug. 10, 2022 /PRNewswire/ — VolitionRx Limited (NYSE AMERICAN: VNRX) (“Volition”), a multi-national epigenetics company, has announced a sponsored research agreement with The University of Texas MD Anderson Cancer Center to evaluate the role of neutrophil extracellular traps (“NETs”) in cancer patients with sepsis.
The study will be led by Jyotika Sharma, Professor of Critical Care Medicine and titled “Correlation of Circulating NETs and Cell Free DNA with Inflammatory Immune Responses in Cancer Patients with Sepsis”. The trial will use Volition’s Nu.Q® NETs test for the study, which was CE marked earlier this year for the detection and evaluation of NETosis.
Dr Jake Micallef, Chief Scientific Officer at Volition, said: “There is an abundance of research into both cancer and sepsis, but relatively few investigations into sepsis in cancer. Cancer patients have a weakened immune system and have a 10 times higher likelihood of developing sepsis and are also more likely to die if they develop it1. Therefore, it is critical for physicians to identify cancer patients at risk of sepsis early and initiate treatment quickly to improve patient outcomes. This is an important study evaluating the potential utility of NETs measurement in the management of cancer patients at risk of sepsis and we’re delighted to collaborate on this research.”
Several publications2-5 report the use of Volition’s Nu.Q® NETs test showing that the high levels of NETs produced in sepsis, COVID-19 and other conditions are associated with, and predictive of, a severe reaction and organ failure.
NETosis is a unique form of cell death that is characterized by the release of NETs, composed of decondensed chromatin, that trap and kill bacteria and viral particles. Although NETs play an important role in our immune system, excessive production can lead to tissue damage and, in severe cases, sepsis, shock, and death.
Volition is developing simple, easy-to-use, cost-effective blood tests to help diagnose and monitor a range of life-altering diseases in both humans and animals. For more information about Volition’s Nu.Q® technology go to: www.volition.com
References
1. https://www.healio.com/news/hematology-oncology/20211117/early-recognition-prevention-strategies-could-reduce-costly-burden-of-sepsis-in-patients-with-cancer
2. https://www.frontiersin.org/articles/10.3389/fmolb.2021.600881/full
3. https://volition.com/media/downloads/Presentations/Rea-et-al-e-Poster-ISTH-2021.pdf
4. https://volition.com/media/downloads/Presentations/Stanford-et-al-e-Poster-ISTH-2021.pdf
5. https://volition.com/media/downloads/Presentations/Published-Poster-Therapeutic-removal-of-NETs-from-blood-in-a-pig-model-of-sepsis-E-ISFA-2021.pdf
About Volition
Volition is a multi-national epigenetics company that applies its Nucleosomics™ platform through its subsidiaries to develop simple, easy to use, cost effective blood tests to help diagnose and monitor a range of life-altering diseases including some cancers and diseases associated with NETosis such as sepsis and COVID-19. Early diagnosis and monitoring have the potential to not only prolong the life of patients but also improve their quality of life. The tests are based on the science of Nucleosomics™, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid – an indication that disease is present. Volition is primarily focused on human diagnostics and monitoring but also has a subsidiary focused on animal diagnostics and monitoring.
Volition’s research and development activities are centered in Belgium, with an innovation laboratory and office in the U.S. and additional offices in London and Singapore.
For more information about Volition, visit www.volition.com
The contents found at Volition’s website address are not incorporated by reference into this document and should not be considered part of this document. The address for Volition’s website is included in this document as an inactive textual reference only.
Volition Enquiries:
Louise Batchelor/Debra Daglish, Volition, mediarelations@volition.com +44 (0)7557 774620
Safe Harbor Statement
Statements in this press release may be “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that concern matters that involve risks and uncertainties that could cause actual results to differ materially from those anticipated or projected in the forward-looking statements. Words such as “expects,” “anticipates,” “intends,” “plans,” “aims,” “targets,” “believes,” “seeks,” “estimates,” “optimizing,” “potential,” “goal,” “suggests,” “could,” “would,” “should,” “may,” “will” and similar expressions identify forward-looking statements. These forward-looking statements relate to, among other topics, Volition’s expectations related to the potential uses, benefits and effectiveness of its Nu.Q® NETs test. Volition’s actual results may differ materially from those indicated in these forward-looking statements due to numerous risks and uncertainties, including a failure by the marketplace to accept Volition’s Nu.Q® NETs test; Volition’s failure to secure adequate intellectual property protection; Volition will face fierce competition and its intended products may become obsolete due to the highly competitive nature of the diagnostics and disease monitoring markets and their rapid technological change; downturns in domestic and foreign economies; and other risks identified in Volition’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as well as other documents that Volition files with the Securities and Exchange Commission. These statements are based on current expectations, estimates and projections about Volition’s business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Forward-looking statements are made as of the date of this release, and, except as required by law, Volition does not undertake an obligation to update its forward-looking statements to reflect future events or circumstances.
Nucleosomics™ and Nu.Q® and their respective logos are trademarks and/or service marks of VolitionRx Limited and its subsidiaries.
View original content:https://www.prnewswire.com/news-releases/volition-announces-us-clinical-study-for-nets-301603353.html
SOURCE VolitionRx Limited

Subscribe for Volition product updates.
Or, click here for Investor Updates and press releases.
"*" indicates required fields